Ⅰ. Introduction
In recent years, bioactive peptides have emerged as highly promising molecules due to their remarkable range of biological activities, particularly in antimicrobial defense. These short amino acid sequences, generated from larger protein precursors, exhibit diverse physiological functions including immunomodulatory, antioxidant, antihypertensive, and antimicrobial properties (1, 2). As global challenges such as antimicrobial resistance and the demand for sustainable biomolecules intensify, bioactive peptides derived from yeast extract offer compelling potential in biomedical, pharmaceutical, and cosmetic.
Yeast extract is a rich source of bioactive peptides that are known to inhibit microbial growth through various mechanisms. It is categorized as growth-phase peptides, fermentation by-products or hydrolysis products, demonstrate antimicrobial and antioxidant properties. These effects are significantly influenced by factors such as pH, temperature, enzyme specificity, and fermentation conditions (3). Their biological activity depends on their structure and amino acid sequence as well as on specific mechanisms of action (2, 4).
Metal-binding peptides (MBPs) have emerged as a promising class of bioactive compounds with the ability to chelate and stabilize essential metal ions, including copper, zinc, iron, and calcium. These peptides, typically derived from enzymatic hydrolysis, contain amino acid residues such as glutamic acid, cysteine, histidine and aspartic acid (5, 6). Coordination of metal ions with peptides often results in improved stability, solubility, and bioavailability, making these complexes more effective as therapeutic agents. In the context of antimicrobial therapy, metal-peptide complexes exhibit potent activity against resistant bacterial strains by generating reactive oxygen species (ROS), disrupting bacterial membranes, and employing multiple mechanisms that reduce the likelihood of resistance development (7).
Among the copper-binding peptide GHK-Cu is a prominent example, known to promote collagen synthesis, tissue repair, anti-inflammatory effects, DNA repair and cell protection (8). However, the therapeutic efficacy of the copper tripeptide GHK-Cu has been limited by its poor permeability through the stratum corneum, which significantly restricts its therapeutic potential. Recent studies have explored the development of novel delivery vehicles that can enhance the dermal penetration and stability of GHK-Cu, aiming to improve its bioavailability and therapeutic potential in clinical contexts (9).
Despite such limitations, copper-binding peptides have gained particular attention due to copper’s critical role in cellular respiration and antioxidant defense mechanisms, offering a biologically compatible strategy to safely deliver copper in a chelated and bioactive form that minimizes oxidative toxicity (10).
The utilization of yeast extract as a precursor for peptide synthesis is further supported by its abundance, cost-effectiveness, and biocompatibility (11). Peptides secreted by Saccharomyces cerevisiae play a crucial role in enhancing the innate immune system through their bioactive properties. These peptides are reported to interact with immune cells, potentially enhancing both innate and adaptive immune responses. The yeast-derived peptides can activate macrophages and increase the production of cytokines (12). Recent studies further highlight the therapeutic applications of copper-peptide complexes, particularly in wound healing, where copper peptide dressings accelerated epithelialization, collagen synthesis and antioxidant defense (13, 14). Their multifunctional properties, including antimicrobial activity and skin regeneration, make copper-binding peptides highly relevant for biomedical and cosmeceutical applications (15).
To address these aspects, the present study focuses on the isolation of antimicrobial peptides from copper-containing biopeptides derived from yeast, with the goal of evaluating their potential for biomedical and cosmeceutical applications.
Ⅱ. Materials and Methods
Yeast-derived peptides, both copper-enriched and non-enriched, were obtained from Macrocare Tech., Ltd. (Ochang, South Korea). Copper enrichment was achieved during fermentation via controlled addition of copper sulfate was performed to allow intracellular accumulation and binding to peptide sequences.
HPLC-grade solvents, reagents, and buffers for peptide extraction, purification, and analysis were sourced from Sigma-Aldrich (Seoul, South Korea). Solvents such as methanol and anhydrous ethanol were purchased from Daejung (Siheung, South Korea), while phosphoric acid (85.0%), trifluoroacetic acid (TFA, 99.0%) and acetonitrile (99.9%), and were obtained from Samchun Chemicals (Seoul, South Korea).
All glassware was sterilized, and reactions were conducted under aseptic conditions. Additionally, microbial strains used for antimicrobial activity screening included Escherichia coli K-12, Staphylococcus aureus 23-394, Bacillus cereus ACCC 04315, Salmonella enterica ACCC 01996, and Candida albicans KCTC 7270. All strains were maintained at −80°C in glycerol stocks and subcultured prior to testing.
The crude extracts of both copper-enriched and non-enriched yeast peptide extracts was carried out at 35°C using reduced pressure in a rotary evaporator. This step was employed to effectively remove additional moisture from the extracts, reducing the possibility of volatile chemicals being lost and maintaining the stability of the peptides for further fractionation processes.
The peptide mixtures were initially separated according to their molecular weight by using size exclusion chromatography (SEC). A Sephadex G-25 gel filtration column (Seoul, South Korea) approximately 2.6 cm by 100 cm was used for SEC. Deionized water was used to pre-equilibrate the column, which was then run at room temperature. The mobile phase consisted of deionized water, and peptide samples (2~5 mL) were carefully loaded into the column and eluted isocratically at a consistent 1.0 mL/min flow rate. UV absorbance at 280 nm was measured to detect peptide-containing fractions. Eluted fractions were collected every two minutes and combined according to comparable retention durations and absorbance patterns.
The Amicon stirred cell filtration system (Sigma-Aldrich, Seoul, South Korea) was used to ultrafilter the concentrated SEC fractions. A set of membranes with molecular weight cut-off (MWCO) values of 30 kDa, 10 kDa, and 3 kDa was used for the process. The process was conducted at 4°C to prevent enzymatic degradation and peptide denaturation. Fractions were centrifuged at 4,500 rpm for 30 min, yielding four molecular size ranges: >30 kDa, 10~30 kDa, 3~10 kDa, and <3 kDa. Each MWCO membrane’s filtrates and retentates were collected separately.
All fractions were promptly frozen at −80°C to maintain their biochemical characteristics, they were freeze-dried for 48 hours using a lyophilizer set at −55°C under vacuum. Until further analysis, the resultant powdered peptide fractions were kept at −80°C in airtight, light-protected containers
The purification process was carried out using RP-HPLC (reverse-phase high-performance liquid chromatography). A photodiode array (PDA) detector on a Young In YL9100 HPLC system (Seoul, South Korea) ensured accurate and sensitive detection. A (250 × 4.6 mm, 5 μm, 12 nm pore size) YMC ODS-A C18 column was used to accomplish the chromatographic separation. System operation and gradient programming were controlled by Empower software (Table 1).
Large-scale purification of bioactive peptides was accomplished using preparative HPLC (prep-HPLC), which was run using YL-Clarity software and a Young In Chromass OBD-A (5 μm, 19 mm × 150 mm) C18 column (Table 2).
The antifungal activity of yeast-derived biopeptides was evaluated against Candida albicans (KCTC 7270). YPD agar plates were overlaid with 6 mL of soft agar (0.7%) inoculated with 1% (v/v) C. albicans culture. A 10 μL aliquot of the peptide sample, adjusted to a concentration of 100,000 ppm, was applied directly to the agar surface. Clear inhibitory zones were observed after the plates were incubated at 30°C for 24 hours.
In a subsequent assay, a disk diffusion-like approach was applied. Paper disks were placed briefly on the agar and then removed to create loading sites, onto which 50 μL of each concentrated peptide sample was deposited and allowed to air-dry. These samples were prepared by pooling residual materials to increase peptide concentration; therefore, the exact concentration was not determined. After 24 hours of incubation at 30°C, antimicrobial activity was evaluated by observing zones of growth inhibition around the treated areas.
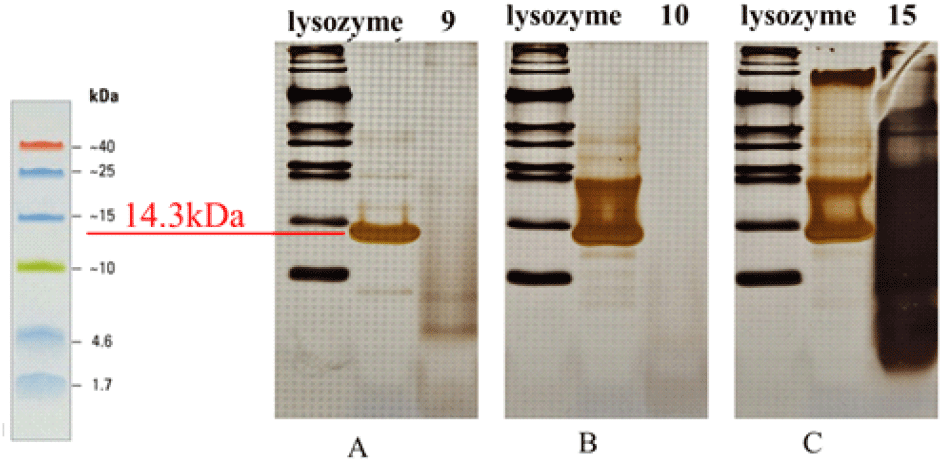
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis, or SDS-PAGE was employed to examine yeast derived peptides that demonstrated antimicrobial activity in previous assays. A 15% separating gel was used for all electrophoresis runs. Only those samples that exhibited a visible inhibition zone in antimicrobial testing were selected for SDS-PAGE. In each well, 20 μL of each sample containing 1,145.4 μg/mL was added. The electrophoresis was conducted under the following conditions: 30 minutes at 95V during the stacking phase and 100 minutes at 120V during the separation phase. Gels were stained with Coomassie Brilliant Blue for 90 minutes and subsequently destained using a solution composed of distilled water, acetic acid, and methanol. The resulting banding patterns were examined for peptide size and intensity.
Ⅲ. Result and Discussion
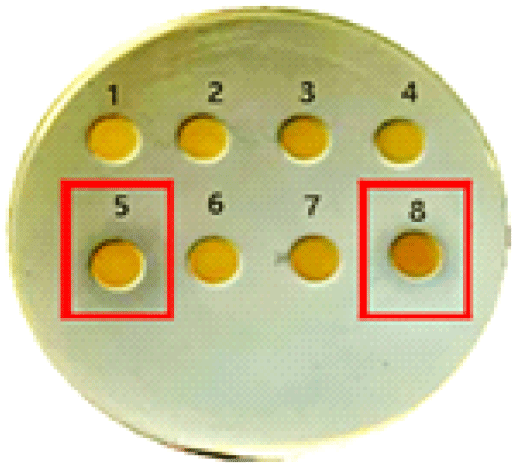
Among the copper-enriched fractions, both the high molecular weight (>30 kDa) and low molecular weight (<3 kDa) exhibited strong antifungal activity, as indicated by prominent zones of inhibition in spot and disk diffusion assays. In contrast, the intermediate fractions (10~30 kDa and 3~10 kDa) displayed weaker inhibition, suggesting a less potent bioactive profile. Surprisingly, there was no antibacterial activity in any of the peptide fractions lacking copper (Figure 1).
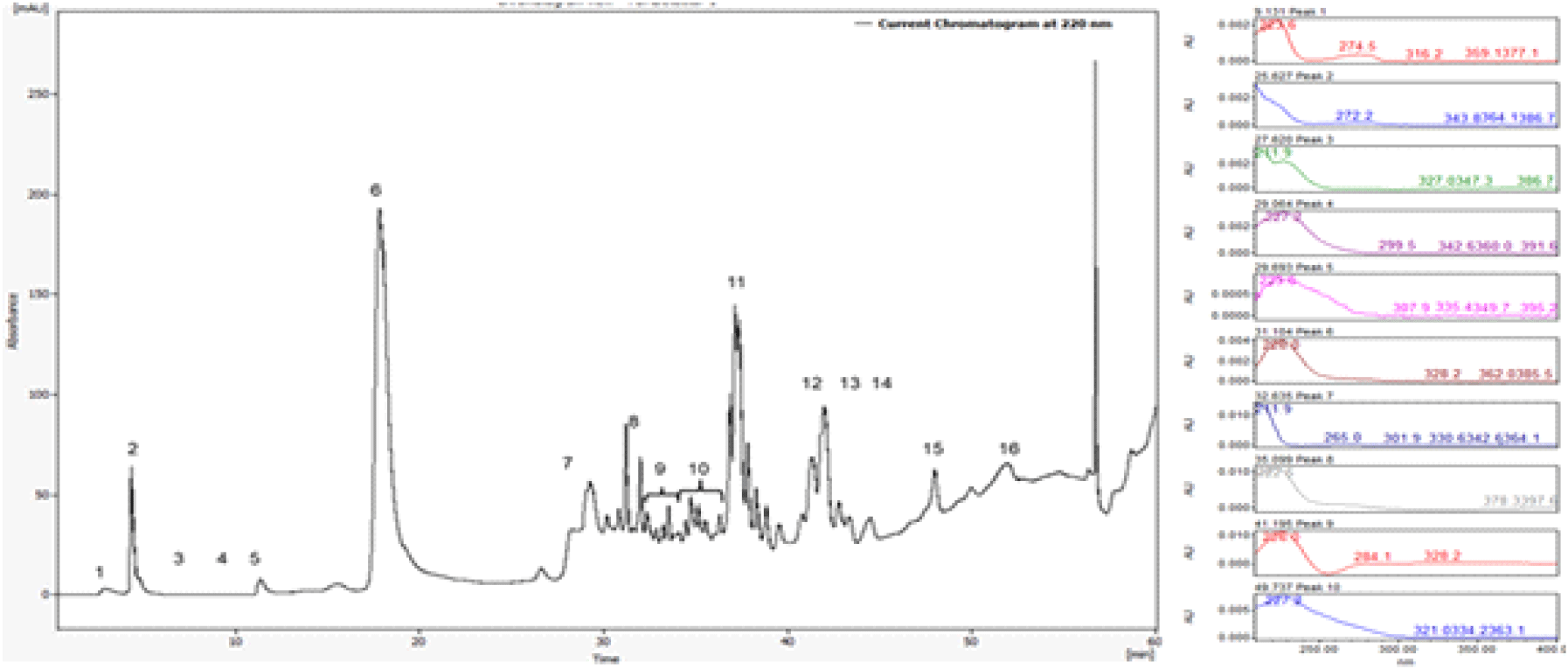
Further purification of the two active fractions (>30 kDa and <3 kDa) was performed using both analytical and preparative liquid chromatography. The preparative HPLC process was optimized using a sample concentration of 140 mg/mL and an injection volume of 2,000 μL to accommodate higher sample loads and enable large-scale purification. Chromatographic separation of the >30 kDa copper-bound fraction resulted in the identification of 16 major peaks, among which fractions 9 and 10 exhibited consistent antimicrobial activity in subsequent biological testing.
To further characterize the eluted fractions, UV-visible spectral analysis was performed across the 200~400nm range. The spectral profiles revealed significant differences among peaks, reflecting variation in their peptide composition and copper-binding properties (Figure 2).
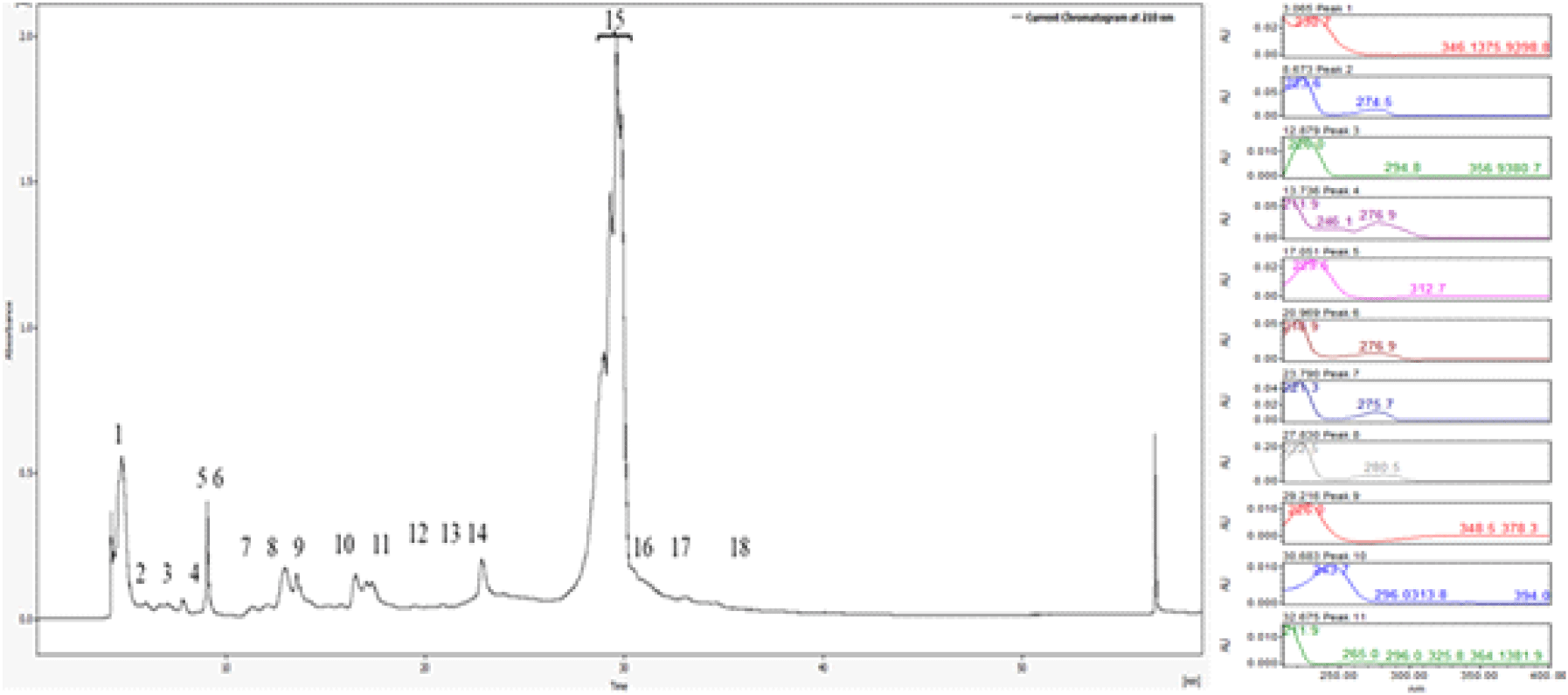
Similarly, the <3 kDa fraction yielded 18 chromatographic peaks, with peak 15 showing strong and reproducible antifungal activity. These bioactive fractions were individually tested using spot assays and disk diffusion methods, confirming their inhibitory effects against Candida albicans. The well-defined inhibition zones surrounding the active fractions provided clear evidence of their antimicrobial potency (Figure 3).
Chromatographic separation was performed on the >30 kDa and <3 kDa copper-bound fractions. Each fragment was tested for antibacterial activity using agar plate assays. Among the >30 kDa fragments, fractions 9 and 10 showed clear antimicrobial activity, while fraction 15 from the <3 kDa group also exhibited antimicrobial effects as shown in Figure 4.
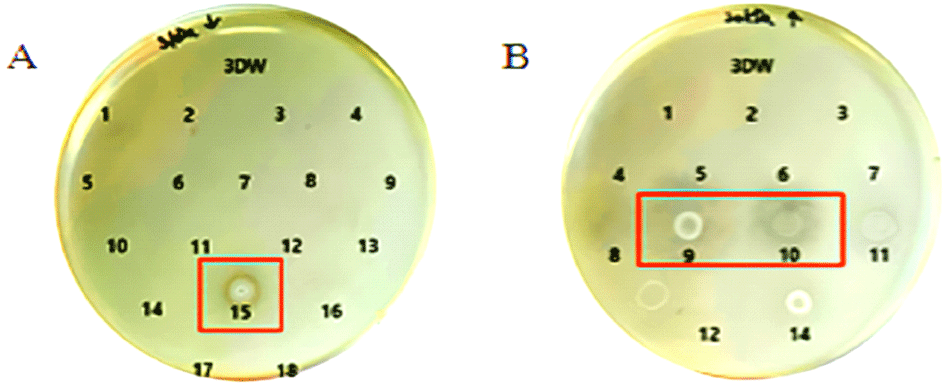
These findings underscore the relevance of molecular size and copper-binding interactions in modulating the bioactivity of yeast-derived peptides. Particularly, the >30 kDa copper-bound peptides appear to harbor more potent antimicrobial components, offering promising candidates for future development of antifungal agents. Further structural and functional characterization of active fractions (e.g 9, 10, and 15) could provide valuable insights into their mechanisms of action and potential therapeutic applications.
SDS-PAGE was performed to evaluate the purified peptide fractions. Line A represents the fraction >30 kDa (fraction 9), and line B represents another fraction >30 kDa (fraction 10). Neither line displayed distinct bands, suggesting that these fractions either contained peptides at very low concentrations, were absent, or had undergone degradation. Line C corresponds to the fraction <3 kDa (fraction 15), which exhibited a prominent band; however, the band migrated downward, indicating possible overloading of the gel or the presence of a very high peptide concentration. For comparison, lysozyme was included as a standard and showed a clear band at ~14.3 kDa, serving as a molecular reference for protein size.
Ⅳ. Conclusions
The present study provides strong experimental evidence that copper-enriched peptides derived from Saccharomyces cerevisiae exhibit potent antimicrobial activity, particularly in the >30 kDa and <3 kDa molecular weight fractions. In contrast, non-enriched yeast peptides have no antimicrobial effects under the tested conditions. These findings confirm that both molecular size and metal coordination are critical factors influencing the biological activity of biopeptides (Figure 5).
Further purification using analytical and preparative HPLC identified specific fractions (>30 kDa: 9 and 10; <3 kDa: 15) with consistent antifungal activity, confirmed by spot and disk diffusion assays. However, SDS-PAGE analysis revealed that bands were not clearly distinguishable in these copper-enriched fractions, likely due to low peptide concentrations, degradation during processing, or limitations of the method. Despite this, the strong biological activity observed highlights the need for additional research to optimize peptide stability, improve detection methods, and further characterize these copper-associated bioactive peptides.
Collectively, this research establishes the foundation for the development of novel, naturally derived, and cost-effective antimicrobial agents suitable for cosmetic, pharmaceutical, or food safety applications.
